22,000% Increase in Deaths following Vaccines for Adults Over 50 as FDA Authorizes 2nd Booster for this Age Group by Brian Shilhavy, Editor, Health Impact News
The FDA announced today that it has issued another emergency use authorization (EUA) for a second COVID booster vaccine for adults over the age of 50, and as young as 12 years old if they are “immune compromised.”
Pfizer had made the request for a second booster shot two weeks ago, but their request was for adults over the age of 65. The FDA, however, lowered that to age 50, and issued the EUA for both the Pfizer and Moderna COVID-19 vaccines.
Amid the ongoing debate over the need for another round of COVID-19 boosters, the FDA has acted quickly on the matter.
Only two weeks after Pfizer and its partner BioNTech asked the agency for an emergency use authorization (EUA) for a second round of COVID-19 boosters in people 65 and older, the FDA has granted the nod. The new FDA authorization covers those who have already been boosted with any COVID vaccine and are either 50 and older or 12 and older if they are immunocompromised.
At around the same time on Tuesday morning, Moderna said the FDA had granted its application for a second booster. The Moderna nod covers adults over 50 who have been boosted once, plus immunocompromised adults over 18. Moderna applied for a second booster on March 17. (Full article.)
Do you think the FDA looked at the data in their Vaccine Adverse Event Reporting System (VAERS) regarding COVID-19 vaccines for this age group to see if there were any concerns before authorizing a second booster shot for this age group?
Not likely.
As we have previously reported, the FDA does no safety oversight on these vaccines, but simply takes the drug manufacturers’ word for it, allowing them to police themselves. See:
Just Released Documents by Pfizer Show BioNTech Paid FDA $2,875,842.00 “Drug User Fee” for COVID-19 Vaccine Approval
So we will review the data on this age group in VAERS as a service to the public.
Here is what VAERS is reporting for people over the age of 50 following COVID-19 vaccines. (Source.)
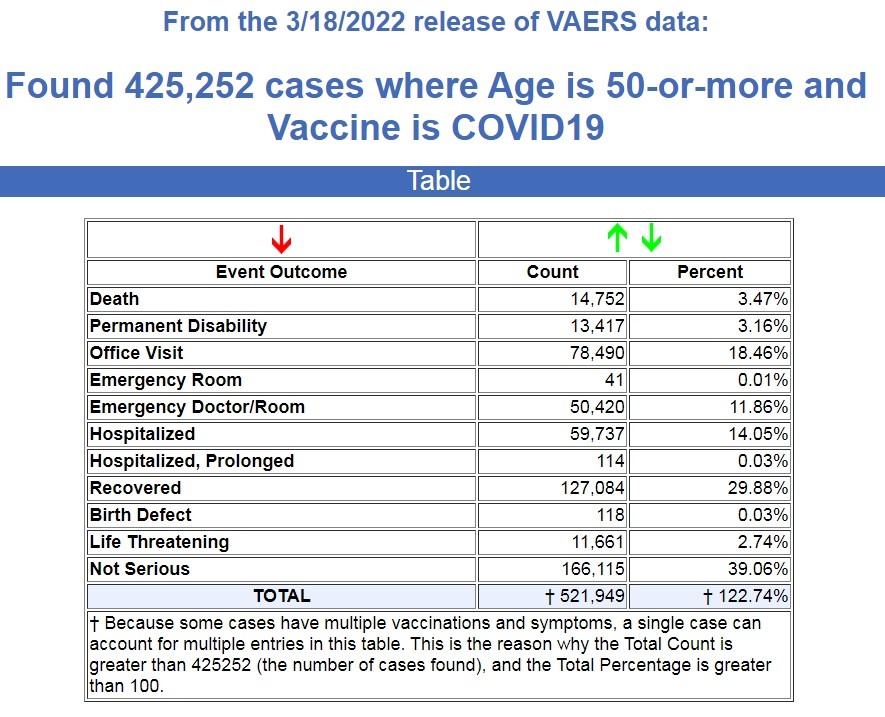
There are currently 14,752 deaths recorded in VAERS for people over the age of 50 following a COVID-19 vaccine, covering a period of 15 months. That’s an average of 983 deaths a month for this age group.
Here are the results for this age group for the previous 30 years following ALL vaccines in VAERS. (Source.)