COVID-19 Vaccine To Be Tested on 6-Year-Olds by Dr. Joseph Mercola for Mercola
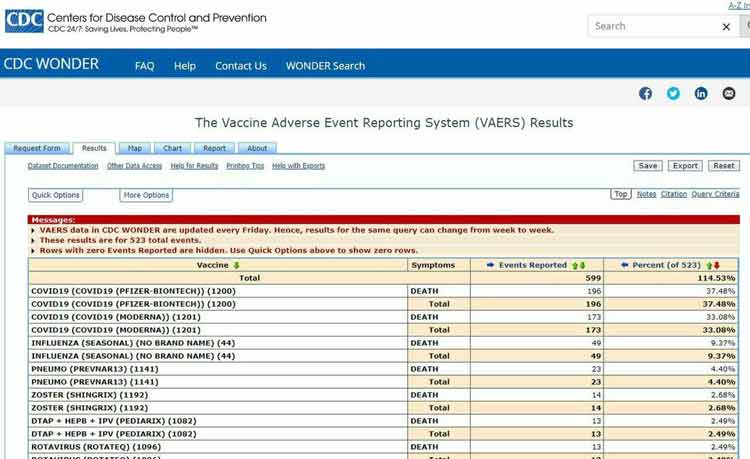
As of February 4, 2021, the U.S. Vaccine Adverse Event Reporting System (VAERS) had received 12,697 injury reports and 653 deaths following COVID-19 vaccination.1
Of the cases reported between December 14, 2020, and February 4, 2021, 3.69% were life threatening and the number of deaths account for 5.14% of the total reports. The Pfizer vaccine accounted for 58% of deaths; Moderna’s accounted for 41%.
What’s more, when you look at vaccine-related deaths between January 2020 and January 2021, you find that COVID-19 vaccines account for a staggering 70% of the annual vaccine deaths, and that’s while having been available for less than two months. The first doses of Pfizer vaccine were given in mid-December 2020,2 while Moderna’s vaccine rolled out during the last week of December 2020.3
While these numbers are staggering, they’re likely only a tiny fraction of the actual number of adverse events. According to a U.S. Department of Health and Human Services study,4 fewer than 1% of vaccine adverse events are ever reported to VAERS.
This is primarily because VAERS reporting is voluntary. Many don’t even know it exists, or that you don’t have to be a medical professional to file a report. This would mean that there may, in reality, be over 1 MILLION COVID vaccine injuries, since 99% typically go unreported.
Report All COVID-19 Vaccine Side Effects
To address these shortcomings and monitor the public health effects of this mass vaccination campaign, the Children’s Health Defense is calling on all who have suffered a side effect from a COVID-19 vaccine to do three things:5
- If you live in the U.S., file a report on VAERS
- Report the injury on VaxxTracker.com, which is a non-governmental adverse event tracker (you can file anonymously if you like)
- Report the injury on the CHD website
Children Are Next
Despite the clear and present dangers of these so-called vaccines, which are in actuality gene therapy, COVID-19 vaccine makers are steamrolling ahead with trials on children as young as 6 years old.
As reported6 by the University of Oxford, which is collaborating on a COVID-19 vaccine7,8 with AstraZeneca, children between the ages of 6 years and 17 years and 8 months are eligible for participation at four U.K. centers. Those over the age of 16 do not even require a parent’s approval but can consent on their own. The remuneration for those putting their entire future at risk is £10 (about $14) per visit.




